Adenocarcinoma, squamous cell carcinoma, and large-cell carcinoma—among other forms of lung cancer—are collectively referred to as non-small cell lung cancer (NSCLC). Those who have undergone Highly Emetogenic Chemotherapy (HEC) and Medium Emetogenic Chemotherapy (MEC) should especially be concerned about chemotherapy-induced nausea and vomiting (CINV). The aim of this work is to evaluate anti-emetic performance in controlling chemotherapy-induced nausea and vomiting. techniques: Ondansetron (OND), Dexamethasone (Dex), Metoclopramide (Met), Ondansetron plus Dexamethasone (OND + Dex), and Aprepitant plus Dexamethasone (Apr + Dex) were five groups broken out from 361 patients. Evaluating the percentage of patients in each group suffering with acute, delayed, and total CINV was the primary goals. After 24 hours or more following therapy, acute and delayed CINV severity were respectively approximated. On chemotherapy day, likert score of 1 or higher for nausea or at least 1 vomiting event; delayed NV was defined as any day between days 1 and 7. Out of 361 individuals, HEC was received by 200 (55.4%) and MEC by 161 (44.6%). Starting within 24 hours after emetogenic drug delivery, HEC medicines generated significantly higher nausea and vomiting (p<0.05) than MEC during the acute period. Whereas vomiting was not statistically different in HEC patients compared to MEC patients, nausea was considerably higher in the delayed phase (>24 hours post-administration) HEC patients (p<0.05). Based on the Likert score, patients utilizing HEC showed improved degree of nausea with time whereas it was worse on the first day. Against acute and delay phases, ondansetron plus corticosteroid combination showed a notable degree of protection. The combinations of aprepitant with corticosteroid, dexamethasone alone (83.7%), and ondansetron alone (83.4%) did not show any appreciable difference (p> 0.05). Based on the results, typical medication regimens to prevent CINV in patients with NSCLC undergoing chemotherapy were ondansetron with corticosteroid combination.
The brain’s vomiting center (VC), located in the medulla oblongata, is responsible for coordinating the vomiting reaction. The emetic reflex is a reaction that the VC induces by integrating a range of peripheral and central signals known as the peripheral and central pathways, respectively [1]. In the peripheral route, stimuli such as gastric/duodenal distension and pharyngeal stimulation are communicated by abdominal vagal afferents [2]. Many receptors, including 5-HT3, neurokinin (NK) 1, and cholecystokinin-1, are expressed by abdominal vagal afferent fibers and can promote the emetic response. The primary mediator of this response is 5-HT3 [3].
Chemotherapy-induced nausea and vomiting (CINV) has been found to be a multifactorial, intricate process involving a wide range of neurotransmitters and receptors. Therefore, the standard of care for preventing CINV in patients undergoing moderately (MEC) or highly emetogenic chemotherapy (HEC) is combined antiemetic regimens that target numerous molecular pathways involved with emesis [4]. These medications induce nausea and vomiting during chemotherapy, both in the immediate 24-hour period after injection (acute emesis) and in the days that follow (delayed emesis) [5]. The fundamental component of antiemetic prophylaxis for both MEC and HEC settings is the combination of a neurokinin-1 (NK1) RA (targeting substance P) and a 5-HT3 receptor antagonist (RA) (targeting serotonin) [6].
The purpose of this study was to assess the safety and effectiveness of antiemetic drugs during both the acute and delayed phases of emesis.
We choose Warith International Cancer Institute, a cancer treatment center authorized by the Ministry of Health in Karbala. The prospective study was accessible to people whose patients were planned to receive MEC or HEC as a first-line cancer treatment. According to the Guidelines for Appropriate Use of Antiemetic Drugs, anticancer medications were classified according to their level of emetogenic risk.
Patients were randomized into five groups: Ondansetron (OND), Dexamethasone (Dex), Metoclopramide (Met), Ondansetron plus Dexamethasone (OND + Dex), and Aprepitant plus Dexamethasone (Apr + Dex). Antiemetic medicines regimens were associated: Ondansetron (A); Dexamethasone (B); metoclopramide hydrochloride (C); Ondansetron plus Dexamethasone (D); and aprepitant with dexamethasone (E).
In order to study chemotherapy-induced nausea and vomiting (CINV) estimation, the complete questionnaires on the same registration form estimating the severity of symptoms in the acute and delayed phases of CINV. Nausea and vomiting that appeared 24 hours or more after the commencement of chemotherapy were classified as acute and delayed CINV, respectively. Additionally, information about the patient’s age, sex, treatment history, use of opioids, use of anxiolytics prior to anticancer drug administration, history of alcohol consumption, history of motion sickness or pregnancy-related vomiting, complete blood count, blood biochemistry results, cancer chemotherapy regimen, and specifics of antiemetic therapy and salvage treatment for CINV were also gathered.
A Likert score of at least 1 for nausea or at least 1 vomiting event on day 1 (i.e., the day of chemotherapy) was used to describe incidents of acute NV, and any day between days 1 and 7 after chemotherapy was used to characterize incidents of delayed NV. Based on the patient investigators assessed vomiting episodes using the National Cancer Institute Common Terminology Criteria for Adverse Events (version 3) [7].
For this study, the analysis was basically descriptive. Descriptive statistics were used to summarize the sample’s demographic and clinical features, the frequency and severity of the delayed emesis, the number of emetic episodes, and the amount of time until the commencement of emesis. To tabulate and compare the incidence of delayed nausea and vomiting in male and female subjects, the chi-squared test was employed. To investigate potential correlations between additional clinical and demographic traits and the total incidence of delayed emesis, a multivariate logistic regression analysis was also conducted.
361 individuals were enrolled in this study between October 2023 and January 2024. The study may have been appropriate for 519 patients in total. 50 patients who chose not to participate, out of the 469 patients who remained present, 73 were not able to be reached by contact for follow-up, and an additional 35 were eliminated due to not having a prescription for delayed antiemetic drugs or not being on study antiemetic regimens. In the end, 361 patients, were included in the study, 200 patients (55.4%) received HEC and 161 (44.6%) received MEC (Table 1).
| Characteristics | % (n) | P Value |
|---|---|---|
| Age (years) | ||
| 21 to 40 years | 28.3 (102) | 0.233 |
| 41 to 60 years | 71.7 (259) | |
| Gender | ||
| Female | 33.2 (120) | 0.421 |
| Male | 66.8 (241) | |
| Marital status | ||
| Single | 15.5 (56) | 0.252 |
| Married | 76.7 (277) | |
| Widowed | 1.9 (7) | |
| Divorced | 5.8(21) | |
| Education level | ||
| No education | 5.3 (19) | 0.006 |
| Primary | 23.3 (84) | |
| Secondary | 44.6 (161) | |
| University | 24.6 (89) | |
| Post-graduate | 2.2 (8) |
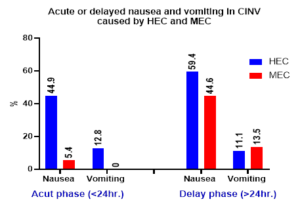
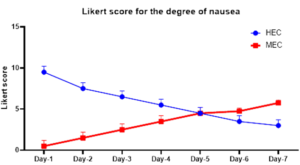
Figure 2 shows the percentage of patients experiencing nausea and vomiting. The outcomes were higher in the delayed phase than in the acute phase. However, we noticed that during in the acute phase, which began within 24 hours after the emetogenic agent administration, HEC medications cause nausea and vomiting significantly (44.9% vs 5.4% and 12.8% vs 0%, respectively, p<0.05) as compared with MEC. While in the delayed phase (> 24 hours after administration); nausea affected 59.4% vs 44.6% of patients receiving HEC vs MEC, that consider significant statically (p<0.05) while there is insignificant difference in vomiting (11.1% vs 13.5%) of patients receiving HEC and those receiving MEC. The degree of nausea was worse on the first day and was better over time in patients taking HEC, according to the Likert score (Figure 3).
The rate of MEC induced acute emesis on day 1 as reported by the patients on their diary card can be seen in Table 2 and Figure 3. Ondansetron plus corticosteroid combination was administered to 95.5% of patients during acute emesis, indicating a high level of protection against this phase. This rate is similar to that of patients treated with Aprepitant plus corticosteroid combination (87.2%). When it came to controlling acute emesis, there was no significant difference (p>0.05) between the combinations of aprepitant with corticosteroid, dexamethasone alone (83.7%), and ondansetron alone (83.4%). Nonetheless, there was insignificant difference (p>0.05) between the acute and delay phases in the management of acute emesis.
\(*P\) value from logistic regression versus others.
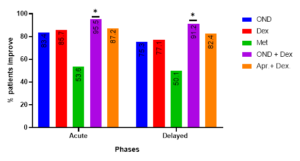
| Category | OND n=41 | Dex. n=30 | Met. n=30 | OND + Dex n=30 | Apr.+ Dex. n=30 | P value |
| Acute (24hr) (day 1) | 83.4 | 85.7 | 53.6 | 95.5* | 87.2 | 0.002 |
| Delayed (24 hr) (days 2–7) | 75.3 | 77.1 | 50.1 | 91.2* | 82.4 | 0.005 |
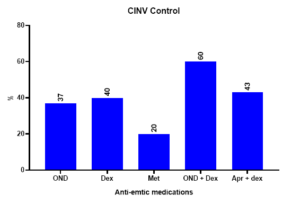
The endpoints of the all-patient groups, complete response, complete protection, and complete control were examined. The findings showed that, for delayed (days 2–7) antiemetic medication, patients that took single medication as Dexamethasone our results showed that there is insignificantly difference (p>0.05) between it and Ondansetron while we found higher proportion of both medications when compared with Metoclopramide to achieve delayed complete control (40% and 37% vs. 20%, P = 0.003). prior to adjusting for measured confounders statistically, patients taking Ondansetron or Dexamethasone alone were approximately 1.3–1.8 times more likely to exhibit delayed complete control than patients taking Metoclopramide (unadjusted odds ratio [OR] = 1.25, 1.83 respectively. 95% confidence interval [CI] = -31.61 to 31.61).
On another hand, we noticed that the combination of Ondansetron with Dexamethasone exhibits higher percent of complete control when compared with Aprepitant plus dexamethasone combination (60% vs 43%) (unadjusted odds ratio [OR] = 1.4. 95% confidence interval [CI] = -11.21 to 21.23).
| Protocol | Admisteration |
Complete
Control |
Complete Protection | Complete Response |
| Ondansetron (A) | MEC n=41 | 15 (37%) | 9 (22%) | 17 (41%) |
| Dexamethasone (B) | MEC n=30 | 12 (40%) | 7 (23%) | 11 (37%) |
| Metoclopramide (C) | MEC n=30 | 6 (20%) | 11 (37%) | 13 (43%) |
| Ondansetron + Dexamethasone (D) | MEC n=30 | 18 (60%) | 4 (13%) | 8 (27%) |
| Aprepitant + dexamethasone (E) | MEC n=30 | 13 (43%) | 8 (27%) | 9 (30%) |
Since CINV is a serious adverse reaction to chemotherapy, accurately estimating the likelihood of its occurrence is crucial. Our research consistently showed that accurately predicting whether a patient will experience CINV after receiving HEC or MEC is challenging. As a result, we recommend following current guidelines and not reducing antiemetics. However, despite confirming previously reported risk factors for CINV, our study did not identify sufficient predictors. We consistently found that younger age was a risk factor for acute nausea, acute vomiting, and delayed nausea, aligning with findings from previous research.
The study’s findings indicate that in the acute phase, approximately half of the patients who received HEC reported CINV, whereas in the delayed phase, more than half of them experienced nausea. According to the Likert scale for the severity of CINV, we observed that the score was high on the first day and gradually declined until the seventh day, while the score was zero during the delay phase and gradually increased until the seventh day, when the maximum score was recorded.
There is still a need for effective treatment of nausea during both the immediate and prolonged periods in patients who receive high emetogenic chemotherapy (HEC) and moderately emetogenic chemotherapy (MEC) [8]. Even though use the single drug such as NK-1 receptor antagonist alone or a 5-HT 3 receptor antagonist or combination, dexamethasone has demonstrated advantages in patients undergoing high emetogenic chemotherapy with cisplatin or cyclophosphamide-doxorubicin [9,10].
We found that combination of serotonin receptor antagonists in combination with DEX had highly efficacy in reduce CINV as compared with other anti-emetics while other studies investigated controversial, Herrington et al. conducted a comparison trial with aprepitant, palonosetron, and DEX alone, There were found that no significant differences in emesis or nausea which aligns with our study findings [11]. Kang et al. found that oral aprepitant, combined with ondansetron, with or without DEX, effectively prevents chemotherapy-induced nausea and vomiting in patients undergoing chemotherapy, compared to controls or those treated only with ondansetron, with or without DEX [12].
The combination of Ondansetron with corticosteroids is considered a standard treatment regimen for preventing CINV in patients with NSCLC undergoing chemotherapy.
We recommended that healthcare providers should using Ondansetron with corticosteroids as part of their treatment guideline for chemotherapy induce CINV to minimize the risk.
Articles are licensed under the Creative Commons Attribution 4.0 International License.
LMJ is hosted by and it is the official publication of the Scientific Committee of the Lebanese Order of Physicians.
Cookies and privacy policy
“we do not share, give, sell, or transfer any personal information to a third party unless required by law”.
ISSN – 0023-8952
E-ISSN – EISSN
Data Last reviewed February .15 . 2011