The primary cause of mortality for people in developed nations is coronary heart disease, or CHD. It is noted that with age the fat deposits in the wall of the coronary arteries as well as the other blood vessels supplying the heart. As a result of this deposition, there is a decrease in the blood supply to the heart causing angina and shortness of breath and may also result in a fatal myocardial infarction. There are several modifiable risk factors for CHD and one of them being the increased level of the amino acid i.e. homocysteine (HCY) which when treated can reduce the risk of CHD. The positive correlation between hyper homocysteinemia and cardio vascular disease (CVD) has established firmly with the data derived from experimental and epidemiological observations. Clinical data authenticate that HCY is an independent risk factor for CVD. The current article is aiming to evaluate potential role of HCY on CVD risk at molecular level, and deep insights into a pathophysiology of CVD and their associations with CVD.
Cardiovascular disease (CVD) refers to the malfunctioning of the heart and blood vessels in the body [1]. Various forms of CVD include stroke, congenital heart defects, hypertension, congestive heart failure, and atherosclerosis, which is the hardening or narrowing of blood vessels, including the coronary arteries [2]. The twentieth century experienced a significant shift in the leading causes of morbidity and mortality, contributing to the global rise in CVD. This phenomenon, known as the epidemiologic transition, impacts individuals of all races, ethnicities, and cultures worldwide, driven by urbanization, industrialization, and associated lifestyle changes. CVD accounts for nearly 30% of global deaths, a figure expected to increase. In 2010, coronary heart disease (CHD) was responsible for 13% of global fatalities and the majority of disability-adjusted life years (DALYs) and years of life lost (YLLs). Stroke was the second leading cause of death, contributing to 11.1% of all deaths and ranking as the third leading cause of DALYs and YLLs globally [3].
Consequently, coronary heart disease (CHD) is the leading cause of death in developed countries. As people age, fat deposits known as atherosclerotic plaques accumulate on the walls of the coronary arteries and the blood vessels supplying oxygen and nutrients to the heart [4]. This buildup restricts blood flow to the heart, potentially causing heart attacks, angina (chest pain usually relieved by rest), and dyspnea [4]. Lifestyle changes can influence many known risk factors for CHD, including physical inactivity, being overweight, smoking, and consuming a high-fat diet. Additionally, elevated blood levels of the amino acid homocysteine are a potentially modifiable risk factor for coronary heart disease [4].
In 1932, American biochemist Vincent DuVigneaud identified a new amino acid when he treated methionine with sulfuric acid. This amino acid was named homocysteine because its structure is similar to cysteine but with one extra carbon atom. Further research revealed that homocysteine acts as an intermediate in the metabolism of sulfur amino acids and transmethylation activities. Before 1962, the biological significance of homocysteine was not understood. It was discovered that children with mental retardation, rapid growth, osteoporosis, dislocated ocular lenses, and frequent thrombosis of arteries and veins excreted homocysteine in their urine. These children often lacked cystathionine synthase, an enzyme dependent on pyridoxal phosphate that catalyzes the conversion of homocysteine and serine into cystathionine. Due to this enzyme deficiency, homocysteine and methionine accumulate to high levels in plasma, while homocystine, the disulfide dimer of homocysteine, is excreted in the urine [5].
An eight-year-old boy’s homocystinuria case that was first reported in 1933 was reviewed in 1969, and it was discovered that a major stroke caused by thrombosis and carotid arteriosclerosis was the cause of death. Moreover, the arteries supplying the body’s major organs were found to be dotted with arteriosclerotic plaques, suggesting a possible connection between homocysteine and atherogenesis.
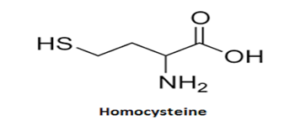
Blood homocysteine levels typically range from 5 to 15 umol/L. It can rise to 50-100 times in cases of illness. Age, vitamin B12 or B6 deficiency, tobacco smokers, alcoholics, and hypothyroidism are associated with a moderate increase. A significant rise in congenital enzyme deficits is observed. Hyperhomocysteinemia (HHcy) is the term for an increase in the level of homocysteine in the blood.
Extremely High Homocysteinemia: Persistently elevated total homocysteine (tHcy) levels (31>100 mmol/L) can result from impairments in B12 metabolism enzymes, MTHFR, or cystathionine beta synthase (CBS).
Slightly elevated tHcy levels (15-30 mmol/L) after fasting may indicate mild enzyme deficiencies, such as thermolabile MTHFR, or decreased homocysteine methylation.
After a Methionine Dose: An abnormal rise in tHcy (>15 mmol/L) following a methionine load of 100 mg/kg suggests defective homocysteine transsulfuration (heterozygous CBS abnormalities, B6 deficiency).
High levels of homocysteine excretion are found in urine. In plasma, there are two forms: homocystine (disulfide, -S-S-group) and homocysteine (with -SH group). Normal urine does not contain either form, but if present, it will contain only homocystine (disulfide). Elevated blood homocysteine levels increase the risk of coronary heart disease. Some data link elevated blood homocysteine levels to myocardial infarction. A rise in blood homocysteine of 5 umol/L is associated with a 20 mg/dl increase in the risk of coronary heart disease. Homocysteine interferes with collagen cross-linking by interacting with the lysyl residues of collagen. It produces a free radical called homocysteine thiolactone, which thiolates LDL particles. These particles tend to clump, are taken up by macrophages, and increase the risk of atherogenesis [6].
Methionine is demethylated to produce homocysteine (Hcy), an amino acid containing a sulfhydryl group. Normally, cystathionine beta-synthase, an enzyme dependent on pyridoxal phosphate, catalyzes the conversion to cystathionine. Additionally, betaine-Hcy methyltransferase and 5-methyltetrahydrofolate-Hcy methyltransferase, two enzymes dependent on vitamin B12, remethylate Hcy to methionine. Plasma homocysteine elevations can result from dietary factors such as vitamin B12 or B6 deficiencies, folate deficiencies, or both. Genetic disorders like methylene-tetrahydrofolate reductase or cystathionine beta-synthase can also cause elevated levels of homocysteine. Numerous factors may be involved in the pathophysiology of Hcy-induced vascular damage, including direct Hcy damage to the endothelium, stimulation of smooth muscle cell proliferation, increased platelet aggregation, enhanced LDL peroxidation, and effects on the coagulation system that lead to capillary damage and cardiovascular disease. Elevated blood homocysteine levels may also represent a modifiable risk factor for coronary heart disease (CHD). Fortifying grains with folate helps lower blood homocysteine levels in the population because the enzyme methylene tetrahydrofolate reductase, encoded by the MTHFR gene, breaks down and removes homocysteine. Pooled data from prospective observational studies that examined the connection between homocysteine levels and the development of CHD showed that folate supplementation, which reduces homocysteine levels, is associated with an 11% lower risk of developing CHD [7].
The molecular basis for the development of arteriosclerotic plaques is linked to the effects of homocysteine on cellular degeneration, damage to the artery intima, cellular proliferation, production of connective tissue, deposition of lipoproteins in plaques, and enhanced blood coagulation. Each of these crucial atherogenesis pathways depends on homocysteine [8].
Elastic fiber degradation and disintegration are other characteristics of early plaques. The internal elastic membrane of arteries fragments due to homocysteine’s activation of the elastase enzyme. Furthermore, homocysteine causes overproduction of collagen in cultured smooth muscle cells, explaining the fibrosis observed in both human and experimental plaques. Additionally, vascular smooth muscle cells proliferate in plaques due to homocysteine’s activation of cyclins, signaling proteins that facilitate cell division. Because homocysteine releases insulin-like growth factor and promotes the sulfation of animal epiphyseal cartilage, it induces smooth muscle cells to proliferate in forming arteriosclerotic plaques and accelerates skeletal growth in children with homocystinuria [5]. The first human study on homocysteine in vascular disease, conducted in 1976, found that dietary methionine increases plasma levels of homocysteine and homocysteine cysteine disulfide in individuals with CHD. Subsequent studies have demonstrated that individuals with peripheral, cerebral, or coronary arteriosclerosis have elevated blood homocysteine levels. An increase in homocysteine is a stronger risk factor than an increase in cholesterol in patients with early onset arteriosclerosis, with an effect akin to that of smoking [9].
There are 2 genes very importantly controls the homocysteine metabolism MTHFR gene (5,10-methylenetetrahydrofolate reductase) situated at 1 p36.3 on chromosome 1 and The 2.2 kilobases long complementary DNA (cDNA) sequence is made up of 11 exons. Two alleles are frequently found with the MTHFR gene. Both the A1298c and C677T alleles.
The primary circulating form of folate, 5-methyltetrahydrofolate, is produced by the enzyme 5,10-methylenetetrahydrofolate reductase (MTHFR), which is important in folate metabolism. The 5-methyl form participates in single-carbon transfers in the production of S-adenosyl-methionine, methylation of proteins, DNA, neurotransmitters, and phospholipids, as well as the remethylation of homocysteine to methionine and nucleotide synthesis. Generally speaking, MTHFR activity helps keep the supply of methionine and folate in the bloodstream and keeps homocysteine from building up [10].
Botto and Yang [9] The MTHFR gene’s position 677, which changes a cytosine (C) to a thymine (T), is the point mutation responsible for the C677T allele. This kind of point mutation causes the enzyme to change its amino acid composition from alanine to valine. The C677T allele is “thermolabile”, meaning that at 37\(^\circ\)C or above, the encoded enzyme’s activity decreases. As a result, compared to similarly treated controls, MTHFR activity in C677T homozygotes is 50–60% lower at 37\(^\circ\)C and 65% lower at 46\(^\circ\)C. Heterozygotes fall into the middle category. When their consumption of folate is inadequate, homozygous individuals for the C677T mutation typically have slightly elevated blood homocysteine levels; when their intake of folate is sufficient, their levels tend to be normal [10].
Botto and Yang [9] the enzyme substitutes glutamate for alanine due to the A1298C allele, a point mutation in exon [7]. The C1289A allele is another name for this allele. While still reduced, the encoded enzyme’s activity is less than that of the C677T allele. Serum homocysteine levels do not seem to be greater in homozygous A1298C individuals than in controls. Nonetheless, individuals with the A1298CIC677T genotype, who are compound heterozygous for the A1298C and C677Talleles, typically exhibit a biochemical profile that is comparable to that of C677T homozygotes, with elevated serum homocysteine and lower serum folate levels [10].
FTO gene alpha-ketoglutarate-dependent dioxygenase, or FTO, is a protein linked to fat mass and obesity. The enzyme known as FTO, which is encoded by the FTO gene in humans and is found on the Fat Mass and Obesity associated gene region (FTO) at 16q10, is highly linked to an increased body weight and an increased risk of developing type 2 diabetes (T2D) [11].
A study conducted on the possible assessment of association of homocysteine with the FTO gene showed that there was a significant increase in the levels of homocysteine by the presence of FTO rs9939609 AA genotype thereby proving that the presence of this FTO gene can alter the levels of homocysteine significantly . Raised homocysteine levels are linked to increased neuroinflammation, hypomethylation caused by decreased Interference with the response of adhesion molecules, B and T lymphocytes, natural killer cells, and S-adelosyl methionine (SAM), an essential methyl donor in a variety of methylation reactions [12]. Future atherosclerosis has been predicted by aortic lipid deposition, which has been linked to hypomethylation. Future atherosclerosis has been predicted by aortic lipid deposition, which has been linked to hypomethylation.
| Source | Age, y | Sample Size | Mean Homocysteine, µmol/L | |||||||
| Cases | Controls | Cases | Controls | P | ||||||
|
[10]
|
21-65 | 99 Cases | 39 Controls |
0.03
0.7 |
0.06
0.6 (P) |
NS
NS |
00000A | |||
| [12] | 69 | 241 Cases | 202 Controls | 5.5 | 4.3 | .001 | 00000A | |||
| [14] | Mean, 62 | 99 Cases | 259 Controls | 13.0 | 10.5 | .001 | 00000A | |||
| [13] | 17-80 | 80 Cases | 22 Controls | 0.76 | 0.40 (P) | .001 | 00000A | |||
The diagnosis of CHD in the four cross-sectional investigations on homocysteine and CHD mentioned above was made using angiographic data showing more than 50% blockage of at least one coronary artery. Blood samples were taken at the time that the diagnosis of CHD was made. While there was no difference in mean homocysteine levels between those with and those without CHD in the first number of trials, those with CHD had higher mean homocysteine levels (between 30% and 90% higher) in the second to fourth number of studies, 10-12. People with high homocysteine levels had significantly higher odds of developing coronary heart disease (CHD), according to all four of the cross-sectional studies that characterized elevated homocysteine levels [13].
The aforementioned [15] case control studies on homocysteine and CHD All but three of the fifteen studies that examined mean homocysteine levels (14, 23, 29) discovered that individuals with CHD had considerably higher homocysteine levels (typically 10%-30%), either after a methionine load or after fasting. Fifteen out of sixteen studies that compared proportions with elevated homocysteine levels found that people with elevated homocysteine levels had an increased risk of coronary heart disease (CHD). The increase in risk was statistically significant at the P\(<\)05. In most of these investigations, the confidence interval (CI) around the RR estimate excluded the null value of 1.0.
RR stands for relative risk; HR for hazard ratio; OD for odds ratio; Prosp = prospective Review-Meta-Analysis = Rev-Meta cross-sectional = cross-sect Randomized control trial, or RCT Heart failure is referred to as HF, coronary heart disease as CHD, cardiovascular disease as CVD, coronary artery disease as CAD, and cerebrovascular disease as Cerebrovasc. NA stands for not available. W stands for women, and M for men.
In above prospective case control , reviews and meta-analysis, randomized control trials ( RCTs) showing that hyperhomocysteinemia is lowering with folic acid \(\pm\) B vitamins and reduces the complications of CVD and stroke , hence these studies are demonstrating an association of hyperhomocysteinemia with an increased CVD and stroke incidences
The authors declare no conflict of interests. All authors read and approved final version of the paper.
All authors contributed equally in this paper.
Articles are licensed under the Creative Commons Attribution 4.0 International License.
LMJ is hosted by and it is the official publication of the Scientific Committee of the Lebanese Order of Physicians.
Cookies and privacy policy
“we do not share, give, sell, or transfer any personal information to a third party unless required by law”.
ISSN – 0023-8952
E-ISSN – EISSN
Data Last reviewed February .15 . 2011