The aim of this study was to assess the quality of some selected ibuprofen products in community pharmacies in Iraq. Different parameters of quality control of pharmaceutical products can guarantee the quality and bioavailability and optimal therapeutic activity. Used quality control parameters, i.e., the variation of weight, friability, disintegration time, dissolution time were tested in vitro. The weight range was (0.56-0.60) g, (0.26-0.33) g, (0.48-0.54) g for Apifen (Ajanta) (400), Apifen (Ajanta) (200), and ibuprofen (flamingo) (400) respectively. Disintegration time was (18min, 17min, and 13min) for Apifen (Ajanta) (400), Apifen (Ajanta) (200), and ibuprofen (flamingo) (400) respectively. Dissolution time at 0.1 N HCL 12.9, 12.2, 18.5, For Apifen (Ajanta) 200 mg, Apifen (Ajanta) 400mg, Ibuprofen 400mg and the dissolution time at Phosphate buffer pH6.8 88.7, 86.6, 90.2 Apifen200mg, Apifen 400 mg, Ibuprofen 400mg. The results showed that all products fulfill the given specification of pharmacopeia (USP-NF) which its dissolution rate was less % in USP (85%after 30min) in phosphate buffer and in 0.1N HCL (18% after 30 min) for ibuprofen(flamingo). Disintegration of ibuprofen 400(flamingo) showed the quickest disintegration while Apifen (400) the slowest.
Ibuprofen is (2RS)-1[4-(2-methyl propyl) phenyl] Propanoic acid Ibuprofen was the first member of Propanoic acid derivatives to be introduced in 1969 as a better alternative to Aspirin. Gastric discomfort, nausea and vomiting, though less than aspirin or indomethacin, are still the most common side effects [1]. Ibuprofen is the most commonly used and most frequently prescribed Non-steroidal anti-inflammatory drugs(NSAIDs) [1-2]. It is a non-selective inhibitor of cyclo-oxygenase-1 (COX-1) and Cyclooxygenase- 2 (COX-2) [3]. Although its anti- inflammatory properties may be weaker than those of some other NSAIDs, it has a prominent analgesic and antipyretic role. Its effects are due to the inhibitory actions on cyclooxygenases, which are involved in the synthesis of prostaglandins. Prostaglandins have an important role in the production of pain, inflammation and fever [4-5]. Ibuprofen is supplied as tablets with a potency of 200 to 800 mg. The usual dose is 400 to 800 mg three times a day [6]. It is almost insoluble in water having pKa of 5.3.8 It is well absorbed orally; peak serum concentrations are attained in 1 to 2 hours after oral administration. It is rapidly bio-transformed with a serum half-life of 1.8 to 2 hours. The drug is completely eliminated in 24 hours after the last dose and eliminated through metabolism [7-8]. The drug is more than 99% protein bound, extensively metabolized in the liver and little is excreted unchanged [9]. Although highly bound to plasma proteins (90-99%), displacement interactions are not clinically significant, hence the dose of oral anti coagulants and oral hypoglycemic needs not be altered [10].
More than 90% of an ingested dose is excreted in the urine as metabolites or their conjugates, the major metabolites are hydroxylated and carboxylate compounds [11-12]. Old age has no significant effects on the elimination of ibuprofen [13]. Renal impairment also has no effect on the kinetics of the drugs, rapid elimination still occur as a consequence of metabolism [14]. The administration of ibuprofen tablets either under fasting conditions or immediately before meals yield quiet similar serum concentrations-time profile. When it is administered immediately after a meal, there is a reduction in the rate of absorption but no appreciable decrease in the extent of absorption [11,15].
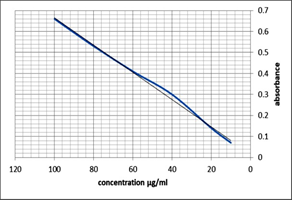
For standard curve of Ibuprofen, first of all stock solution was prepared by taking 20 mg of Ibuprofen in 100 ml solvent (phosphate buffer pH 6.8) in a volumetric flask. The drug was dissolved in the solvent by magnetic stirrer. Five different dilutions were prepared of the stock solution (0.2 mg/ml) having concentrations, 0.1 mg/ml, 0.05 mg/ml, 0.025 mg/ml, 0.0125 mg/ml and 0.0062 mg/ml respectively. These prepared dilutions were then analyzed by UV-Visible Spectrophotometer at 264 nm. The values of absorbance were plotted against concentrations and a slope was developed as given in Figure 1.
A-Weight variation: For each brand, 20 tablets were randomly selected and weighed individually using an analytical balance. The average weights were calculated and the percentage deviations from mean values were calculated. Then the standard deviations, and percentage of related standard deviation (RSD)was determined [16].
B-Hardness test: Hardness test Tablet hardness is defined as the force required breaking the tablet in a diametric compression test. If the tablet is too hard, it may not disintegrate in the required period of time to comply with the dissolution specification. Conversely, the hardness must not be so low that the tablets are soft and friable. To get a satisfactory quality tablet hardness should be between 4 and 8 kg 6 [17].
C-Friability test: It is defined as the percentage of weight loss of powder from the surface of the tablets due to mechanical action and the test is performed to measure the weight loss during transportation. in friability test the tablets are prone to abrasion hence enabling us to check for the tablet strength under application of force in different manner [18].
D-Disintegration time test: Six tablets were subjected to this test. One tablet was introduced into each tube of disintegration apparatus with addition of a disc. The assembly was suspended in the beaker containing distilled water 37oC and the apparatus was operated for 15 minutes. The assembly was removed from the liquid and detected for any fragment of tablet remains.
E-In vitro dissolution rate studies: Paddle dissolution Apparatus was used for the in vitro drug release studies of Ibuprofen, the study was carried according to the, USP method. Two different Medias were used. The dissolution tests were performed at 50 rpm, in 900 mL of 0.1 N HCl, phosphate buffer pH 6.8, at 37C (3 replicates). The drug dissolved is determined by UV absorbance (Lambda 264 UV) on filtered portions of the dissolution media. The percentage of drug released after 30 min was recorded using the linear regression equation of the calibration curve. These test in the tablet and suspension [19].
The weight range was (0.56-0.60) g, (0.26-0.33) g, (0.48-0.54) g for Apifen (Ajanta) (400), Apifen (Ajanta) (200), ibuprofen (flamingo) (400) respectively, as shown in the table below.
| Brand | Minimum weight(g) | Maximum weight(g) | Average weight(g) |
Standard
Deviation (SD) |
%Relative
Standard (RSD) |
| Apifen (400) | 0.56 | 0.60 | 0.571 | 0.008 | 1.046 |
| Apifen (200) | 0.26 | 0.33 | 0.292 | 0.009 | 1.401 |
| Ibuprofen(flamingo) (400) | 0.48 | 0.54 | 0.494 | 0.010 | 1.402 |
Disintegration time was (18min, 17min, and 13min) for Apifen (Ajanta) (400), Apifen (Ajanta) (200), and ibuprofen (flamingo) (400) respectively. Dissolution time at 0.1 N HCL 12.9, 12.2, 18.5, For Apifen (Ajanta) 200mg, Apifen (Ajanta)400mg, Ibuprofen 400mg and the dissolution time at Phosphate buffer pH6.8 88.7, 86.6, 90.2 Apifen200mg, Apifen 400mg, Ibuprofen 400mg.
| brands | hardness | friability | Disintegration time(min) |
Dissolution test (%)of
drug released |
|
| 0.1N HCl |
Phosphate
buffer pH= 6.8 |
||||
| Apifen200mg | 6.59±0.2 | 2.9±0.4 | 17±0.2±0.1 | 12.9±0.2 | 88.7±0.1 |
| Apifen 400mg | 13.4±0.1 | 2.85±0.2 | 18±0.1±0.3 | 12.2±0.1 | 86.6±0.4 |
| Ibuprofen400mg | 12.75±0.1 | 0.54±0.4 | 13±0.4±0.1 | 18.5±0.3 | 90.2±0.1 |
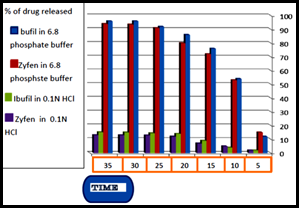
Ibuprofen is one of the commonly used medicines as it is effective, available, and reasonably priced, and it has multiple pharmaceutical forms, so it can be used for all ages. This is what prompted us to make an evaluation of some of the products we have available to several companies. We used the conventional evaluation methods for grains, and our results show the best product and give expected result is ibuprofen400 (flamingo) depending on pharmacopeia (USP-NF). We saw the dissolution test is the most important way to study, under in vitro conditions, the release of a drug from a solid dosage form, and thus represents an important tool to determine factors that affect the bioavailability of a drug from a tablet dosage form. It is an evaluation as to whether or not a tablet releases its drug contents when placed in the environment of the gastrointestinal tract. The rate of dissolution may thus be directly related to the efficacy of the oral solid dosage form, as well as to bioavailability differences between formulations. The test is conducted using a specially designed instrument (dissolution apparatus) with selected pharmacopeia specification. The dissolution performance of ibuprofen if as tablet or suspension is tested when poured in the dissolution vessel only 20% of the active dissolve at low PH in 60 min whereas ibuprofen dissolves in few minutes in PH6.8 buffer, confirming that the solubility of ibuprofen strongly depends on the PH of the solution. The second important thing we used in evaluation two different companies for two suspension and this only method can use in evaluation of suspension and saw the ibuprofen dissolution quickly when the acidity low. Depending on the result we should protect the ibuprofen from the acid of GI to get good dissolution and disintegration therefore good bioavailability product.
Ibuprofen is a commonly prescribed analgesic and anti-inflammatory drug. Currently many generic and multinational brands of this drug are available in the Pharma market in the gulf region. it has been observed that multi sourcing of a drug product might lead to variability in clinical responses and eventually dissatisfaction among prescribers and consumers. Small differences in manufacturing process, different formulation factors such as type and amount of excipients, packaging or storage factors and substandard as well as counterfeit products could alter the disintegration, dissolution and other parameters that consequently lead to variation in therapeutic response. The results showed that all products fulfill the given specification of pharmacopeia (USP-NF) which its dissolution rate was less % in USP (85%after 30min) in phosphate buffer and in 0.1N HCL (18% after 30 min) for ibuprofen(flamingo). Disintegration of ibuprofen 400(flamingo) showed the quickest disintegration while Apifen (Ajanta) (400) the slowest.
We advise any person to do the search about ibuprofen to sure from availability of matter because the difficulty that we faced when search about the active constituent of ibuprofen. The old devices second problem we faced you made us repeat the test more than once so we advise the researchers to use more development devices for get more accurate results.
Articles are licensed under the Creative Commons Attribution 4.0 International License.
LMJ is hosted by and it is the official publication of the Scientific Committee of the Lebanese Order of Physicians.
Cookies and privacy policy
“we do not share, give, sell, or transfer any personal information to a third party unless required by law”.
ISSN – 0023-8952
E-ISSN – EISSN
Data Last reviewed February .15 . 2011